Seeing ME/CFS: Creating the First Mouse Models for the Disease
"Many scientists and clinicians think ME/ CFS is not real – that it’s some sort of “yuppie disease”. So, we went to back to the basics. We asked ourselves “Why do they think ME/ CFS is not real”. Our answer was "because they don’t see it”. So, we set out to show it."
Avik Roy, PhD (Chief Scientific Officer of Simmaron Research Inc.)
Thus, Simmaron Research Foundation researchers set out to build a model of ME/CFS so glaringly clear that no one could dispute it. In doing so they're focusing on producing one of the big missing pieces in the ME/CFS field - a mouse model.
The goal: enable researchers to see and better understand ME/CFS by creating an ME/CFS mouse model.
I think we all have feelings about using mice or any other animal to understand diseases. Mice, however, play an important and even crucial role in medical research and drug development.
Look up mouse models in the PubMed medical research citation database and you'll find them virtually everywhere. Hundreds, if not thousands of mouse models have been created for cancer alone. Multiple mouse models have been produced for heart failure, lupus, Sjogren's Syndrome, Alzheimer’s disease, multiple sclerosis - you name it - every major disease has produced mouse models that mimic different aspects of the disease. The Jackson Laboratories maintains over 9,000 genetically defined strains of mice.
Mice are good stand-ins for humans in several ways. Sharing 80% of their genes, they’re biologically quite similar to humans and come down with many of the same diseases. Extensive research into mice means we know their genes and physiology inside and out. Because we can genetically manipulate mice to mimic virtually any disease that occurs in humans establishing a mouse model allows researchers to explore a disease in exquisite detail.
The overlap mice share with humans also allows them to play a critical role in drug development. Because mouse models allow researchers to rapidly test many different compounds for efficacy not having a mouse model cuts ME/CFS out of a huge swath of drug development opportunities. Plus, when it comes to drug development the FDA expects to see animal model data before it will allow a new drug to be tested in humans. Jackson Labs reports that a "mouse strain with relevant disease symptoms provides a primary, effective and efficient model that is vital to the process of drug discovery."
Gulf War Illness Mouse Model Spurs Research
Mouse models play an important role in medical research and drug development. The creation of a Gulf War Illness mouse model, for instance, provided novel insights and supported the development of a large clinical trial
The recent development of a Gulf War Illness mouse model may help uncover what could be in store for ME/CFS when and if an animal model is fully developed. First the GWI mouse model showed how even a relatively brief exposure to chemicals in combination with a highly stressful event could conceivably result in decades (in human terms) of cognitive problems.
Another GWI mouse model study concluded that the inflammation in GWI is found exclusively in the brain; i.e. it showed that GWI is primarily a neuroinflammatory disease – an important distinction. GWI researchers next used mouse models to identify molecular pathways in the brain that had been altered. Lower levels of some omega-3 and omega-6 essential fatty acids, phospholipids, and cardiolipin - a fat that is specific to the mitochondria - pointed to damaged membranes and problems with energy production.
Recently GWI mouse models have also been used to understand the gut issues in GWI and assess new possibilities for healing them. Dr. Klimas subsequently used GWI mouse models to test her supercomputer models of GWI. That successful test enabled her get funding for a clinical trial to test her unique two-drug approach to GWI. Those results enabled her to get enough private funding for a similar trial in ME/CFS.
While mice obviously can't be complete stand-ins for humans there are clearly many reasons why mouse models continue to play such a large role in medical research and drug development - and why ME/CFS has been missing out by not having one.
The Simmaron Research Foundation’s ME/CFS Mouse Models
Simmaron researchers are working to create two ME/CFS mouse models: a transgenic and a chemical-induced model. As we’ll see simply the process of creating an ME/CFS mouse model has resulted in significant potential insights.
First, though, they had to get the process going. Researchers using animal models must implement what’s called the 3Rs (Replacement, Reduction, Refinement) to minimize the harms to the animals, and make sure they’re only used in scientifically significant work in high-quality scientific projects.
The first step was, in collaboration with the University of Wisconsin-Milwaukee UWM, acquiring an animal laboratory and behavior suite that could easily assess fatigue; i.e. record the animal’s activity levels as well as their cognitive functioning.
Wanting more direct assessments of muscle fatigue, they got creative and designed their own unique treadmill-EMG recording device to assess muscle electrical activity (eg muscle functioning) during exercise. After the Animal Care Committee at UWM approved their protocols, and with the help of a donation from a Canadian Foundation, they began what may be the first animal model study in ME/CFS.
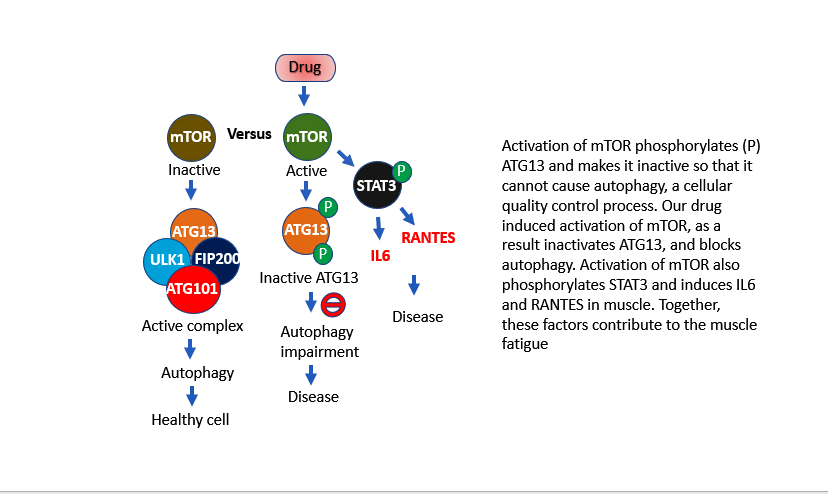
The strain of mice they used– called B6 – is the most commonly used inbred mouse and was the first to have its entire genome sequenced. They gave the mice a compound , an mTOR activator and autophagy inhibitor that inactivates a protein called ATG13 which activates two mytokines called IL-6 and RANTES. Although, IL-6 is released by the muscles during exercise and is believed to enhance energy production, together with RANTES it can cause molecular changes in muscle tissue causing fatigue
The Simmaron mice, then, were given a drug that impairs energy production and oxygen consumption during exercise – two problems studies have shown are present in ME/CFS.
Interestingly, the mice displayed a dramatic gender split with the female mice much more apt to display fatigue – a sign given the gender split in ME/CFS that they were on the right track. Electromyography (EMG) muscle tests revealed that bicep muscles in the mice given the autophagy inhibitor quickly became fatigued, and their grip strength declined. Likewise, a treadmill test indicated they were less active and displayed increased fatigue. More testing is needed but thus far, they look very much like ME/CFS mice. They’re bringing in more mice to confirm the findings.
I asked the Simmaron team what’s next?
Their ultimate goal is uncovering the molecular factors of fatigue in ME/CFS. Is it neurogenic; i.e. is it caused by a nervous system impairment or is it myogenic; i.e. caused by damage to the muscles?
Roy stated that their next project involves making a transgenic mouse model of ME/CFS; i.e. a mouse model into which human genes have been introduced that they believe will produce some of the cardinal symptoms of ME/CFS such as PEM and OI.
Producing a “transgenic” ME/CFS mouse model; i.e. mouse into which human genes have been added is next.
Given the extensive mouse data created for two other movement and fatigue affecting diseases - Parkinson’s Disease and multiple sclerosis – they’re also interested in understanding how ME/CFS is different from them.
Finally, they’re planning to test a series of drug compounds they’ve called SIMMPYRA (Simmaron; Provisional patent application) that they’ve applied patents for.
This is one of a series of blogs charting the transformation of the Simmaron Research Foundation’s ME/CFS research effort and its goal to redefine how ME/CFS is understood, studied, and treated.
Find out more - Innovation - A Hallmark of Simmaron’s New Research Team
Be a part of creating these tools that are essential to drug development for ME/CFS. Support Simmaron and its effort to uncover better treatments for this disease.